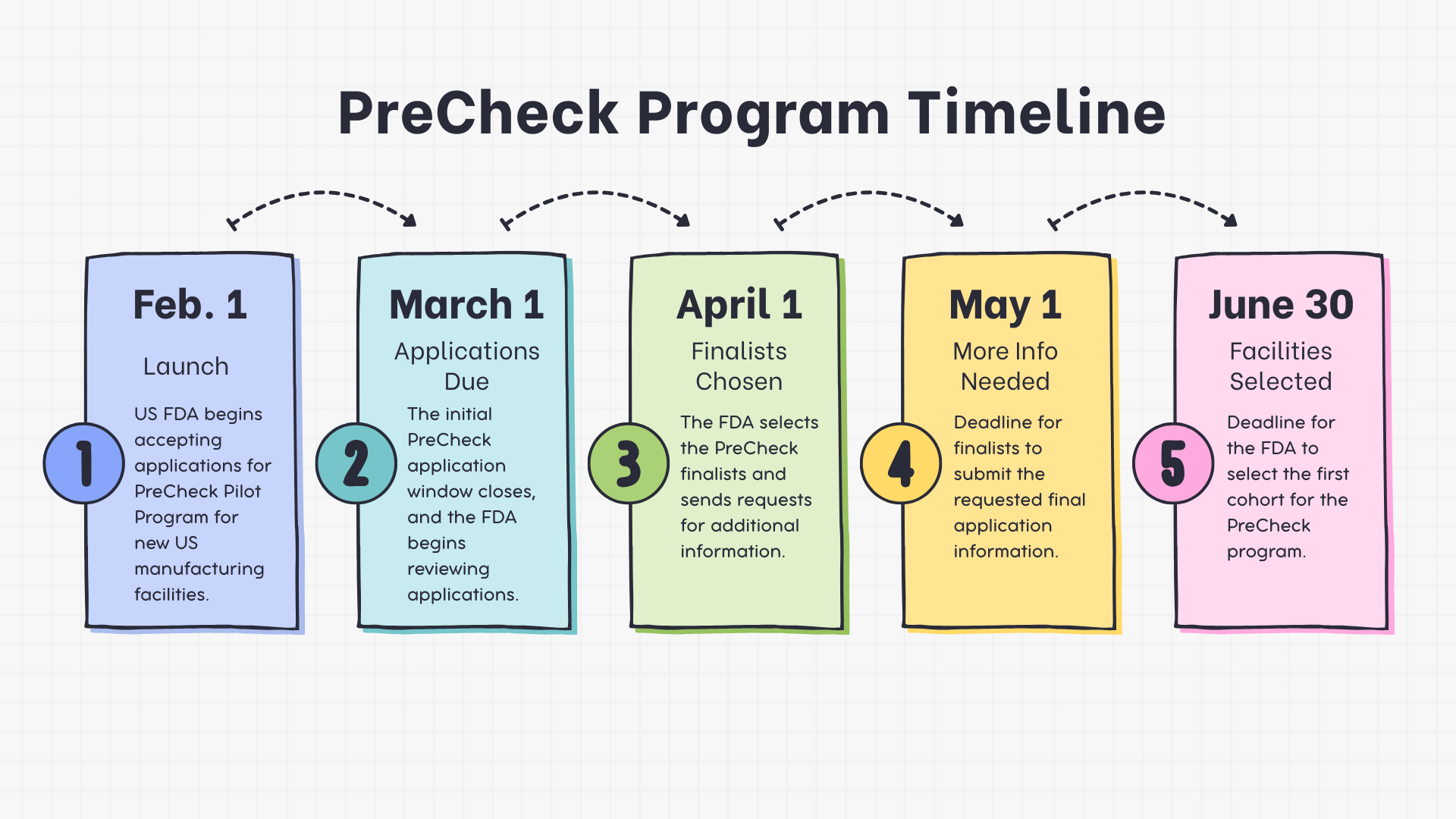
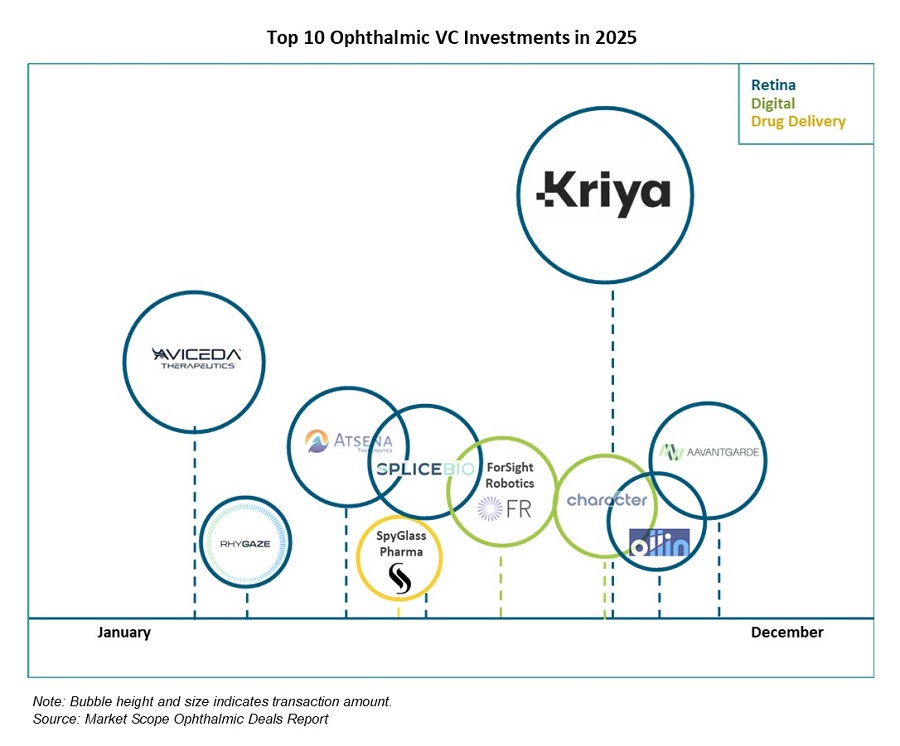
More News

2025 and Quarterly Ophthalmic Revenue Roundup for Amgen, Santen, Hoya, Lumibird, and Ocular Therapeutix
Amgen, of Thousand Oaks, California, reported Feb. 3 that its 2025 revenue for Tepezza was $1.90 billion, a 3 percent increase over $1.85 billion in 2024, primarily driven by higher net selling pri...
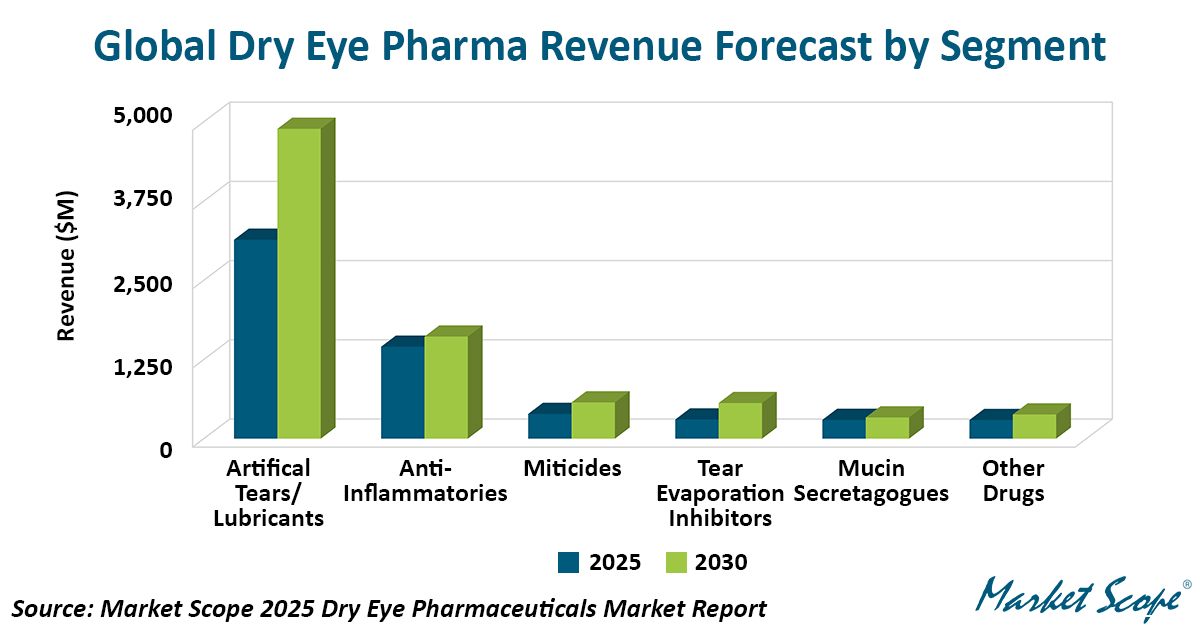
Three New Prescription Drugs Reshape the US Dry Eye Pharmaceutical Market, According to Market Scope
Three recently launched prescription therapies are rapidly redefining the competitive landscape of the US dry eye pharmaceutical market and are expected to drive significant revenue growth over the...
Want to Read Locked Articles?
Already Have an Account?
Register A Corporate Account
A corporate account gives you access to licensed reports and subscriptions, the latest news, a personalized dashboard, and weekly emails with news and data.